Same Day Shipping. Quick Response. Call us at 800.232.2403.

Fast, Accurate Screening Solutions
For more than a decade, NexScreen has been the leader in premium drug screening products, offering fast, effective and reliable drug screening cups and other testing products.

Same Day Shipping on Most Orders
Custom Configurations Available
Volume and Industry Discounts Available
Shop
Premium drug, alcohol, and nicotine testing products and controls.
Trusted By

Automate the Drug Screening Process
Save time, eliminate common errors, and reduce reporting delays with the i3ccf drug screening collection system. Get a fully customizable all-in-one software portal built for your business and streamline your testing process.
On a single platform you can order and manage:
- Conveniently schedule a rapid drug test from any device
- Real time status updates
- No More Paper CCFs
- Electronic Capture of Signatures
- MRO Expedited Adjudication Process
- National Prescriptive Authority

One Login For
Employment Screening Services
Employment Health Screening
Contractor Screening Services
Verified Results
NexScreen Drug Screening Products
Trusted Reliability, Superior Accuracy

Rapid Urine Drug Test Cup
CLIA Waived and FDA 510(k) Cleared
- As accurate as a lab test
- Results in 5 minutes
- Unbreakable, tamper proof, leak proof
- 13 drug and 6 adulterant screens
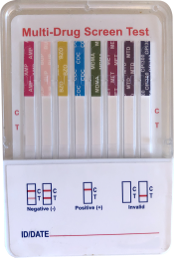
Urine Dip Card Drug Test
CLIA Waived and FDA 510(k) Cleared
- Quick and easy to use
- Results in about 1 minute
- As accurate as a lab test
- Up to 12 panel test configurations

Oral Fluid Drug Tests
CLIA Waived and FDA 510(k) Cleared
- Easy to collect
- Detect recent drug use
- No gender-specific observation
- Up to 10 panel test configurations

